RGUHS Nat. J. Pub. Heal. Sci Vol No: 12 Issue No: 2 pISSN: 2249-2194
Dear Authors,
We invite you to watch this comprehensive video guide on the process of submitting your article online. This video will provide you with step-by-step instructions to ensure a smooth and successful submission.
Thank you for your attention and cooperation.
1Dr. Dhanya PR, Assistant Professor, Department of PG studies in Kayachikitsa, Shri Shivayogeeswara Rural Ayurveda Medical College and Hospital, Inchal, Karnataka, India
*Corresponding Author:
Dr. Dhanya PR, Assistant Professor, Department of PG studies in Kayachikitsa, Shri Shivayogeeswara Rural Ayurveda Medical College and Hospital, Inchal, Karnataka, India, Email: dhanyapr984@gmail.com
Abstract
Background: Constipation is one of the most common gastrointestinal diseases. The contemporary management with laxatives has several drawbacks. Thus, a randomized controlled trial was designed to evaluate an Ayurvedic polyherbal formulation in comparison with a control group.
Objective: The study was conducted to evaluate the efficacy of an Ayurvedic polyherbal formulation, Chitraka granthikadi kashaya, in the management of clinically diagnosed primary constipation.
Methodology: The current study was a prospective, randomized, open-labelled, active-controlled, parallel group, clinical study, with pre and post-test design. It was conducted at SSCASR-Bangalore, Karnataka between May 2021 and May 2022. A computer generated table (block) randomization technique was adopted. A total of 30 subjects, aged 21- 65 years, diagnosed with constipation were assigned to two groups - Control [Group A; n=15], received the control drug Isabgol husk powder, 3.5 g twice daily, after food and the Intervention group [Group B; n=15] received trial drug Chitraka granthikadi Kashaya, 5 mL twice daily, before food for 20 days. Subjective parameters were assessed based on the assessment criteria at baseline, 20th day and drug free follow-up on 27th day.
Results: The trial was completed with 30 subjects [no dropouts]. Both groups demonstrated clinically significant improvements in subjective parameters.
Conclusion: A statistically significant result was obtained in both the groups; however, Chitraka granthikadi kashaya showed a sustained reduction in the incidence of constipation.
Keywords
Downloads
-
1FullTextPDF
Article
Introduction
Constipation is one of the most common gastrointestinal conditions among the public globally. The International Classification of Diseases (ICD10-CM-K59) defines constipation as the decrease in normal frequency of defecation accompanied by difficult or incomplete passage of stool or passage of excessively hard, dry stool.1
The prevalence of constipation is estimated to be 16.8% nationally, while global estimates range from approximately 1% to 80%.2 According to different epidemiological studies, the prevalence of constipation tends to increase with age and has shown greater incidence in females than in males.3
The major risk associated with the contemporary management of constipation includes drug dependency as well recurrence of symptoms.4 This makes it a major yet ignored public health issue.
Chitraka granthikadi kashaya, an Ayurvedic polyherbal formulation,5 is described to have Vatahara (Alleviating vata- a body component responsible for movement as per Ayurveda), Agnideepana (Enhancing digestion), Amapachana (Removing the accumulated toxins), Anulomana (Correcting the downward movement), Rechana (Laxative) properties. Its popular usage as a Vibandhahara dravya was hypothesised in the current study owing to these potent properties of all the dravyas individually and in combination.
Materials and Methods
Study design and study site
A 27-day, single centred, prospective, randomized, active-controlled, open-label, comparative, parallel group clinical study was conducted at Sri Sri College of Ayurvedic Science and Research, Bangalore, Karnataka.
The study subjects were randomly recruited from the OPD, IPD of the hospital, and the special camps conducted.
Ethical-approval, consent-and clinical trial registration
Ethical clearance was obtained prior to the study from the Institutional ethics committee with ethical clearance certificate number - SSIEC/130/2020.
An informed consent form was created in conformity with WHO research methodology guidelines and a bilingual consent form was created and approved by Institutional ethics committee. The consent was taken from each participant enrolled for the study. The trial was registered prospectively in Clinical Trials Registry of India (CTRI No. CTRI/2021/03/031740).
Objectives of the study
To analyze the efficacy of Chitraka granthikadi kashaya in the management of constipation.
Inclusion criteria
Participants aged 21-65 years, who met the diagnostic criteria for constipation according to the updated Rome IV criteria for constipation, were eligible for inclusion in the study.6
Exclusion criteria
Subjects with uncontrollable systemic ailments and neurological ailments, subjects on medication that might cause constipation, lactating and pregnant women were excluded from the study.
Withdrawal criteria
Subjects with acute illness requiring emergency management and subjects who were not willing to continue the study were considered as withdrawal criteria.
Intervention
Group A (Control drug)
Oral administration of the control drug, Isabgol husk powder, 3.5 g twice daily after food for 20 days.7
Group B (Trial drug)
Oral administration of the trial drug, Chitraka granthikadi kashaya, 50 mL fresh preparation twice daily, before food with 1.5 g Saindhava lavana for 20 days (Table 1).
Method of preparation of Kashaya churna
Dried raw drugs of Chitraka moola (Plumbago zeylanica L.), Pippali (Piper longum L.),Erandamoola (Ricinus communis L.), Shunti (Zingiber officinale Roscoe.) were taken and powdered coarsely. Equal quantities of these coarse powders were then measured and mixed together.
Then, a packet of 250 g Kashaya churna along with 60 g Saindhava lavana was made ready for the final dispensing.
Method of preparation of Kashaya
The subjects were instructed to prepare the Kashaya by following the prescribed method: 12.5 g of Kashaya churna taken along with 200 mL of water. Then it was boiled and reduced to 1/4th i.e, 50 mL Kashaya.8 This boiled Kashaya was then filtered and the participants were advised to take it with 3 g of Saindhava lavana.9 Each Kashaya packet was provided with sample measurements to ensure clarity and avoid any sort of confusion during preparation.
Outcome measures evaluation
Assessment was done for the subjective parameters on every assessment schedule, including the drug free follow-up on 27th day. Bristol stool form scale was used for assessing the consistency, and other subjective parameters were assessed based on the Rome IV criteria which included frequency of defecation, straining, and satisfactory defecation.
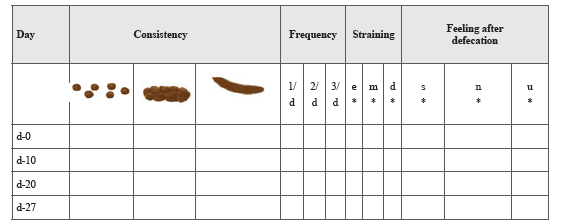
Assessment schedule: 0th,10th 20th, 27th day (follow-up) (Figure 1).
This figure is based on the Bristol stool form for consistency of stool. E- indicates easy evacuation. M - indicates mild stress. D- indicates defecation with stress. S- indicates satisfied defecation. N- indicates not fully satisfied. U- indicates unsatisfied. The instructions to follow for assessment criteria includes (a) What is the consistency of the stool, is it a well-formed or hard or very hard. (b) What about the daily evacuation of the stool, is it once in a day or once in 2 days or once in 3 days or more. (c) What is the nature of defecation, is it easy or mild stress for defecation or do you require straining for defecation. (d) What about your satisfaction levels after defecating, are you completely satisfied or not fully satisfied or unsatisfied defecation.
NOTE: These questions are intended to ask to participant by the investigator. Based on their reply, the investigator have to fill this.
Sample size determination
The sample size was calculated using the appropriate sample size estimation of an RCT10 and was estimated to be 120. However, due to the short duration of the study, a sample of 30 subjects was randomized, with 15 subjects in each group. The estimated duration of the trial was one year.
Randomization technique
An online block randomization method with a pre-generated random number was used for randomization, with a balanced allocation ratio 1:1. Following initial screening and enrolment, participants received the intervention according to a pre-fixed allocation sequence using block randomization and subject numbers. The study participants were unaware of the randomization and allocation measures.
Statistical analysis
The data obtained were systemically arranged and statistical analysis was performed. For the assessment of non-parametric values, Wilcoxon signed rank test and Friedmans test was applied within the group and Mann Whitney U test was applied between the groups. The effect size was calculated to ascertain the clinical efficacy. Statistical analysis was done using SPSS software IBM SPSS Statistics for Windows, Version 26 (Armonk, New York: IBM Corp.). The corresponding P value was noted and obtained results were interpreted as-Statistically non-significant for P value >0.05.
Statistically significant for P value <0.05.
Statistically highly significant for P value <0.01.
Results
Demographic data
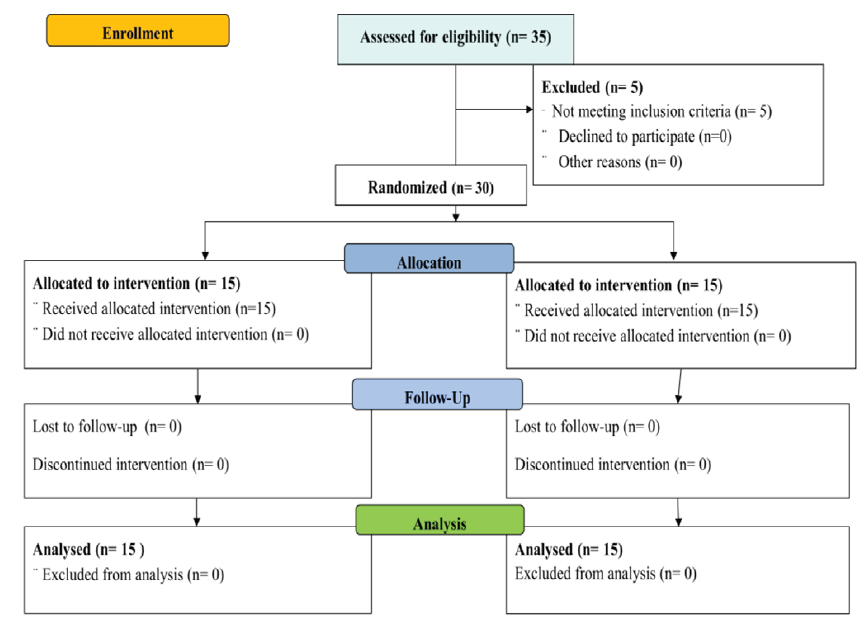
The CONSORT flow diagram template is presented in Figure 2. The study was completed with 30 subjects, without any major or minor protocol deviations. Among the study subjects, it was observed that the maximum number of subjects i.e., 13 (43.33%) were in the age group of 51-60 years and 63.33% of subjects were male. A majority of the subjects i.e., 50% belonged to lower middle class. 83.32% of the subjects were from Jangala sadharana desha and Jangala desha.
About 20% of participants reported bloating of abdomen as an associated complaint. 73.33% subjects followed a vegetarian diet, while 56.66% indulged in alpamatra ahara, 43.33% indulged in sama ahara and 56.66% consumed madhura pradhana ahara. Around 66.66% indulged in vishamashana, 50% reported irregular exercises, 56.66% reported having the habit of consuming coffee, 66.66% subjects consumed ruksha ahara, 43.33% subjects reported reduced water intake. Around 70% of the total study subjects followed a sedentary life style. About 63.33% did vegadharana, 36.66% reported day time sleeping habit. 43.33% subjects belonged to vata-kapha prakruti, 50% subjects had mandagni and the remaining 50% were of vishamagni (Table 2, Table 3).
Adverse drug reaction (ADR) & Rescue medication
The reporting form for suspected adverse reactions mentioned by the Pharmacovigilance of Ayurveda, Siddha, Unani and Homoeopathy (ASU & H) drugs was used for any suspected ADR’s during the study period. No adverse drug reaction was noticed during the study period.
Rescue medication
No rescue medication had to be used as there was no aggravation of symptoms in any of the study subjects.
Statistical results
Subjects were recruited for the study between May 2021 and May 2022. Statistical analysis was done for all the subjective parameters such as, consistency, frequency, straining and satisfaction after defecation, to understand the efficacy of the interventions.
The mean scores of the measured parameters within each group showed comparable results. As a decrease in majority of the parameters was observed in both the groups, a between group comparison was conducted to determine which group demonstrated a greater effect or whether the responses were similar. Additionally, effect size was calculated to provide a more meaningful interpretation of the clinical outcomes.
When the effects of the medicine on the symptoms were compared between the control and trial groups, the outcomes were found to be similar.
Discussion
Incidence and etiology
Previous studies performed in larger populations indicate that prevalence of constipation rises dramatically with age.11 Similar observation was drawn from the present study as well. This might be attributed to the lack of physical activity and unhealthy dietary practices prevalent in this age group. Although it is well documented that females are more prone to developing constipation,12 the present study does not support this finding. This discrepancy may be due to a higher proportion of male participants in the study.
The higher prevalence of constipation among well-educated individuals of the society could be due to the prolonged physical inactivity, which is a known etiological factor for constipation in otherwise healthy individuals. This highlights the impact of a sedentary lifestyle on the development of constipation.13
Socioeconomic status was calculated based on the modified Kuppuswamy scale and it was found that 50% of the subjects belonged to lower middle class. This might be an indicator of higher prevalence of constipation in people belonging to the lower socioeconomic strata of the society, likely due to a reduced intake of dietary fibre, quality compromised diet and unhealthy lifestyle.14
According to Koppen climate classification system, the geographical areas of the subjects were categorised and the corresponding desha were identified.15A higher incidence of participants from the Jangala sadharana desha was observed, which may be due to the study being conducted in such a region.
A previous study done to investigate the effect of physical activity on constipation showed a higher incidence in physically inactive subjects.16 Dietary components have the capability to modulate both the composition and, more importantly, the function of the intestinal microbiome. This implies that the majority of study subjects consumed diets high in simple sugars, fats, and fast foods, with low cellulose content, factors that may have contributed to their difficult bowel habits.17
The consistency of stools help to identify the intestinal transit and degree of constipation. Some studies have reported that stool water content is correlated to stool hardness and also to the insoluble calcium fatty acid soaps (formed from calcium and fat, especially the long-chain fatty acids in the intestine).18 A study revealed that reduced intestinal transit time and physical activity are associated with increased stool frequency.19
Another study confirmed that sensation of satisfactory bowel emptying in sitting defecation posture necessitates excessive expulsive effort compared to the squatting posture. This might be the reason for the unsatisfactory and straining during defecation reported by majority of the subjects in the present study.20
Some researchers suggested that even minor disturbances in gut microflora can lead to significant changes in gut function, including gas production. Although the overall volume of gas production may not significantly change from individual to individual, the content (methane [CH4], hydrogen [H2], or carbon dioxide [CO2]) may vary greatly, potentially leading to changes in intestinal transit and visceral sensation. This might be the reason for bloating which was an associated complaint in the present study.21 It is well established that the prevalence of psychological disorders is significantly higher among individuals with constipation compared to the general population.22
The quantity of ahara an individual consumes depends upon the Agni bala.23 Ruksha is the guna of vata; so the intake of more ruksha pradhana ahara can cause vataprakopa.24
Studies revealed that, caffeine being the prime ingredient of coffee and tea acts as a stimulant and ease the bowel movements by increasing the colonic motility. However, alongside, it can also cause dehydration.25 This dehydration effect might be the reason for constipation in majority of the subjects.
Discussion on results
Discussion on consistency of stool
The consistency of stools was assessed based on the bristol stool form chart during each assessment schedule. The change in the consistency of stools from very hard to well-formed was observed in majority of subjects, with a statistically highly significant result in control group. This indicates the efficacy of Isabgol husk powder in improving the stool consistency. An increase in the mean value during the drug-free follow-up period indicates a recurrence of symptoms upon discontinuation of the medication. However, in the trial group receiving Chitraka granthikadi kashaya, a gradual reduction in the mean value was observed across all the assessment intervals, suggesting a consistent improvement in stool consistency, from hard to well-formed. To correlate the statistical significance with clinical improvement, effect size was determined and was found significant. Thus it can be concluded that, on comparing the results of consistency of stool between the groups, trial group (Chitraka granthikadi kashaya) showed a better result than control group in reducing hard consistency of stools, both statistically and clinically with a sustained and persistent effect.
Discussion on frequency of stool
The frequency of stool was assessed based on the assessment criteria before the treatment and on every consecutive assessment schedule. An increase in the frequency of stool after treatment, with recurrence of symptoms during the drug-free follow-up in the control group was noted, suggesting improvement in frequency only with the administration of medicine. However, in the trial group, a gradual decrease in the mean value indicates a progressive reduction in symptoms across consecutive assessment schedules. When comparing the results for frequency of defecation between the groups, a significant improvement was observed with sustained and persistent effect on increasing the defecation frequency. This effect was found to be more efficient in Group B than Group A, both statistically and clinically.
Discussion on straining during defecation
Straining during defecation was assessed before the treatment and at every follow-up appointment. Reduction in straining was observed in majority of subjects with statistically highly significant results in the control group. This indicates the efficacy of Isabgol husk powder in reducing the straining during defecation. But an increase in the mean value during the drug-free follow-up in the same group indicates the recurrence of symptom on discontinuation of medication. However in the trial group, a gradual reduction in the mean value was observed during all the assessment schedules and it implies the gradual reduction in straining during defecation.
Thus it can be concluded that, comparing the results related to straining during defecation between the groups, trial group was found to be superior than control group in reducing the straining during defecation, both statistically and clinically with sustained and persisting effects.
Discussion on satisfactory defecation
Satisfactory defecation was assessed before the treatment and at each follow-up, for all the subjects using pre-defined assessment criteria. In the control group, improvement in satisfaction after defecation was observed in majority of subjects with a statistically highly significant result. This indicates the efficacy of Isabgol husk powder for satisfactory defecation. But an increase in the mean value during the drug-free follow-up indicates the recurrence of symptom on stoppage of medicine. In the trial group, a gradual reduction in the mean value was observed during all the assessment schedules implying a gradual improvement from unsatisfactory to satisfactory levels of defecation. So it can be concluded that, on comparing the results regarding satisfactory defecation between the groups, trial group showed a better outcome than control group, both statistically and clinically with a sustained and persistent effect.
Limitations
Due to limited financial resources and time constraints, the current study was conducted on a small sample size. This represents a major limitation, as the findings may not be sufficient to draw conclusive inferences applicable to the general population. Therefore, future studies with larger sample sizes, a multicentre approach, and more robust study designs are recommended.
Recommendations
Further studies involving a larger population are warranted to establish more conclusive and generalizable results. Future studies can be conducted to understand the efficacy of Chitraka granthikadi kashaya in other types of constipation owing to the positive effect demonstrated by the drug in the present study. Stool culture can be performed to analyse the gut microbiota, and this might be helpful in identifying the bacterial species that promote the development of constipation. Colorectal transit studies can be performed to analyse the transit time. Colonic manometry can be done, as it provides reliable information regarding the pathophysiology of constipation and can be used to explore the mechanisms and effects of pharmacological agents on the colon.
Conclusion
The study demonstrated clinically and statistically significant results in managing the symptoms of constipation. Although both treatments were beneficial in managing constipation, Chitraka granthikadi kashaya showed greater benefit with a more sustained effect compared to the control drug. Thus, based on the clinical outcome, from the present study, it can be hypothesised that Chitraka granthikadi kashaya is superior to Isabgol husk powder in managing constipation. However, further studies involving larger sample sizes are warranted to establish more conclusive and generalizable results.
Source of Funding
Nil.
This research has not received any special grant from funding agencies in public, commercial, or not for profit sectors.
Acknowledgement
I thank Dr. B. Ravishankar, Ex-director, SDM Centre for research in Ayurveda and allied sciences, Ex-Head of pharmacology laboratory, IPGT & RA, Gujarat Ayurveda University, Jamnagar for his continuous support and guidance. All the faculty members in the Department of PG-Studies in Kayachikitsa, Sri sri college of ayurveda science and research, Bengaluru for all their support. thank Farmers pharmacy pvt. Ltd Kerala for providing the raw drugs. I also thank Dr. Umesh chikkanna, Department of Integrative Medicine, National Institute of Mental Health and Neuro Sciences- Bengaluru for his guidance.
Conflict of interest
None.
Supporting File
References
1. The International Classification of Disease. TheWeb’s Free2019/2020 ICD-10-CM/PCS Medical coding reference[Internet]. Geneva; ICD10data.com;2007 [updated 2007; cited 2020 Jan 5]. Available from: https://www.icd10CM/ codes/k00-k95/k55-k64/k59-k59.00 [accessed 2020 Feb 18]
2. Ray G. Evaluation of the symptom of constipation in Indian patients. J Clin Diagn Res [Internet]. 2016 Apr [cited 2020 Feb]; 10(4):OC01-03. Available from: http://www.ncbi.nlm.nih.gov/m/ pubmed/27190857.
3. Forootan M, Bagheri N, Darvishi M. Chronic constipation:A review literature.CCBY-NC-ND [Internet]. 2018 May [cited 2020 Feb10];97(20):e10631. Available from:https://www.ncbi.nlm.nih.gov/pmc/ articles/pmc5976340/#!po=68.229
4. Porwal A, Gandhi P, Jaya M, et al. An open–label, prospective clinical study to evaluate efficacy of constac in the management of chronic constipation. Int J Ayu Pharm Res [Internet]. 2015 Mar 6 [cited 2020 Feb 12];6(6):756-59. Available from: http:// ijapr.in/index.php/ijapr/article/view/308
5. Vagbhata. Ashtanga hridaya of Vagbhata, Arunadutta krit. Edited by Vaidya Jadavji Trikamji Acharya. Chikitsa sthana. Ch.14, Ver. 48. Varanasi: Chaukhamba Sanskrit Sansthan; 1990. p. 225
6. Sarpodar S, Bhor S, Bhor IS. Non-parametric methods. In: Ranade S, Deshpande RR, editors. Research methodology and medical statistics. Ch. 11, 3rd ed. Pune: Manikarnika Publication; 2015. p. 252-59.
7. Peter WD, Sykes J. A multi-centre, general practice comparison of ispaghula husk with lactulose and other laxatives in the treatment of simple constipation. Curr Med Res Opin [Internet]. 1998 [cited 2020 Feb 10];14(4):227-33. Available from: https://pubmed.ncbi.nlm.nih.gov/9891195/
8. Dalhana, Commentator. Susruta Samhita of Susruta. Chiktsa sthana. Ch. 39, Ver. 8.Varanasi: Chaukhamba Sanskrit Sansthan; 1990. p. 509.
9. Sreeradhakrishnaparasar, editor. Sarangdharasamhita of Acharya Sarangadhara.Madhyamakhanda, Ch.2,Ver.5.6th edition.Nagpur: Sreevaidyanathayurvedbhavana; 2012. p.74.
10. Daniel WW, Cross CL. Biostatistics: A foundation for analysis in the health sciences. 10th ed. Hoboken (NJ): John Wiley & Sons, Inc.; 2013. p. 192.
11. Gandell D, Straus SE, Bundookwala M, et al. Treatment of constipation in older people. CMAJ 2013;185(8);663-670.
12. Verkuijl SJ, Meinds RJ, Trzpis M, et al. The influence of demographic characteristics on constipation symptoms: a detailed overview. BMC Gastroenterol 2020;20(1):168.
13. Iovino P, Chiariani G, Giancarlo B, et al. New onset of constipation during long-term physical inactivity: A proof-of-concept study on the immobility-induced bowel changes. PLoS One 2013;8(8):e72608.
14. Wani RT. Socioeconomic status scales-modified Kuppuswamy and Udai Pareekh's scale updated for 2019. J Family Med Prim Care 2019;8(6):1846- 1849.
15. Arnfield, AJ. Koppen climate classification [Internet]. Encyclopedia Britannica; 2020 [cited 23 March 2022]. Available from: https:// www.britannica.com/science/Koppen-climate-classification
16. Sayad AT, Dalia MK, Walid K, et al. Effects of a proposed physical activity and diet control to manage constipation in middle aged obese women. Diabetes Metab Syndr Obes 2017;10:513-519.
17. Lee BR, Ko YM, Cho MH, et al. Effects of 12-week vegetarian diet on the nutritional status, stress status and bowel habits in middle school students and teachers. Clin Nutr Res 2016;5(2):102-111.
18. Matsuda K, Akiyama T, Tsujibe S, et al. Direct measurement of stool consistency by texture analyzer and calculation of reference value in Belgian general population. Sci Rep 2021;11:2400. Available from: https://doi.org/10.1038/s41598- 021-81783-7
19. Panigrahi MK, Kar SK, Singh SP, et al. Defecation frequency and stool form in a coastal eastern Indian population. J Neurogastroenterol Motil 2013;19(3):374-80.
20. Sikirov D. Comparison of straining during defecation in three positions: results and implications for human health. Dig Dis Sci 2003;48(7):1201-5.
21. Lacy BE, Gabbard SL, Crowell MD. Pathophysiology, evaluation, and treatment of bloating: hope, hype, or hot air? Gastroenterol Hepatol 2011;7(11):729-739.
22. Hosseinzadeh ST, Poorsaadati S, Radkani B, et al. Psychological disorders in patients with chronic constipation. Gastroenterol Hepatol Bed Bench 2011;4(3):159-163.
23. Sharma RK, editor. Charka Samhita of Agnivesha. Sutrasthana, Chapter 5, Verse 3-4. 1st ed. Varanasi: Chaukamba Sanskrit Series; 2012. p. 105-106.
24. Sharma RK, editor. Charka Samhita of Agnivesha. Sutrasthana, Chapter 12, Verse 4. 1st ed. Varanasi: Chaukamba Sanskrit Series; 2012. p. 235.
25. Iriondo-DeHond A, Uranga JA, Del Castillo MD, et al. Effects of coffee and its components on the gastrointestinal tract and the brain-gut axis. Nutrients 2020;13(1):88.